Teagasc phase 1 research analysis into the beneficial properties of TonicTreat
Aim of Innovation Voucher
The aim of this work was to assess the nutritional and potential bioactive or health benefits of MeatSnax products – 4 in total – made using different botanical extracts and formulations known only to the commercial producer. Bioassays that demonstrate the potential heart health (ACE-1 inhibitory assay) benefits of feed ingredients, anti-inflammatory activity (COX inhibition) of ingredients and potential to inhibit Type 2 Diabetes (DPP-IV inhibitory) were carried out. In addition, nutritional analysis was carried out on the final formulated products.
The following samples were received from MeatSnax on the 26th February 2020:
- 06202001 – Original & Seaweed sample for Analysis (Sample 3)
- 07202001 – Antiworm + sample for analysis (Sample 4)
- 0820001 – Dental + sample for analysis (Sample 2)
- 07202001 – JointAid – Sample for analysis (Sample 1)
- Bioactivity Assays
Samples were stored at room temperature until further use. A number of bioassays were performed on the samples.
Assay 1: Heart health relevant – Angiotensin-1-converting enzyme (ACE-1-inhibitory) activity
Background: Angiotensin-converting enzyme (ACE-I) inhibitors are widely used to treat chronic cardiac heart failure (CHF) in dogs and cats. In the pathogenesis of cardiac heart failure (CHF), the proteolytic enzyme renin is released by the kidneys and acts on angiotensinogen, which is produced by the liver and distributed in the blood, to produce angiotensin I. The formation of angiotensin II from angiotensin I occurs through the action of ACE. Angiotensin II causes retention of Na+ and water, in part through stimulation of the synthesis and release of aldosterone by the adrenal cortex. Angiotensin II also causes vasoconstriction, thus increasing systemic vascular resistance. ACE also results in degradation of bradykinin and, thus, ACE inhibitors lead to increased levels of bradykinin that contribute to their vasodilatory effects. By inhibiting the formation of angiotensin II, ACE inhibitors prevent vasoconstriction and reduce retention of Na+ and water in animals with CHF. ACE inhibitors are balanced vasodilators, reducing both preload and afterload. The effects during CHF include decreased vascular resistance and cardiac filling pressures and increased cardiac output and exercise tolerance. However, ACE inhibitors have only a mild effect on afterload reduction and should not be used as monotherapy in animals with severe systemic hypertension (>160 mmHg). ACE inhibitors are also frequently used (typically in combination with other arterial dilators) to manage systemic hypertension in dogs and cats. Somewhat paradoxically, ACE inhibitors such as benazepril have been shown to be beneficial in treatment of some forms of renal disease. Enalapril is approved in the USA to treat CHF secondary to DCM and MMVD in dogs. Benazepril is approved in several countries other than the USA to treat CHF in dogs. It is difficult to get animals to consume medication and food derived ACE-I inhibitory agents might provide an option for pet owners to manage blood pressure specifically hypertension in dogs.
Did ACE-I inhibition assay on 13/07/2020

To calculate the % ACE-I inhibition the absorbance of B1 and B2 are important. The positive control Captopril was used in the assay.
% ACE-1 inhibition = (Abs blank 1 (cell A) – Abs sample)/ (Abs blank 1 – Abs blank 2) X 100

% ACE-1 inhibition by samples (Meat Snax)
Figure 1: Inhibition of ACE-1 enzyme by meat snax products
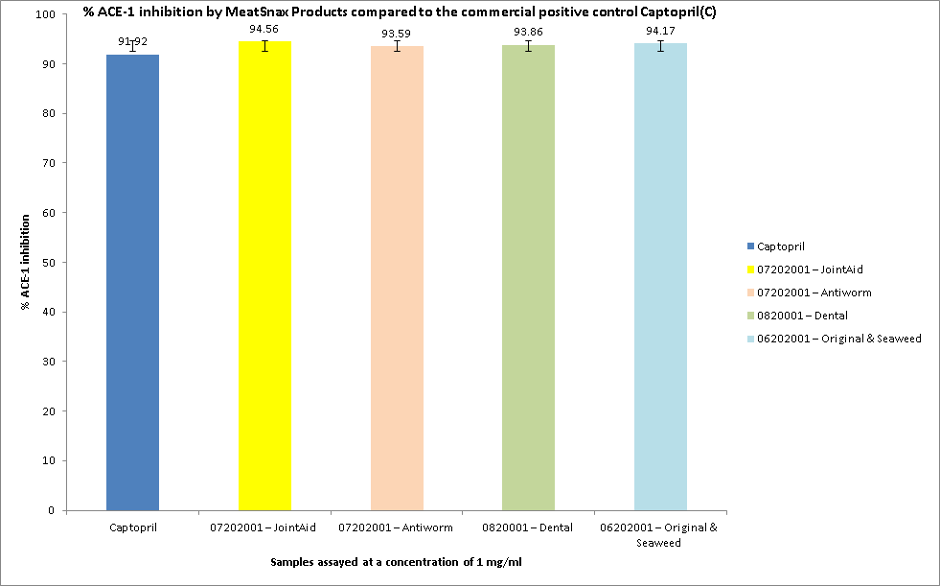
Result: As shownin Figure 1, excellent ACE-I inhibitory activities was observed when the MeatSnax products were tested at a concentration of 1 mg/ml in vitro compared to the positive control Captopril® tested at a concentration of 0.5 mg/ml. The assay was carried out in triplicate and ACE-I inhibition ranged from 93.59 % for the antiworm meat snax product to 94.56 % ACE-I inhibition for the Joint Aid product when tested at a concentration of 1 mg/ml in vitro compared to the positive control Captopril® which inhibited ACE-I by 91.92 % when assayed at a concentration of 0.5 mg/ml, respectively.
Further work will involve determining the IC50 values for the JointAid product.
Assay 2: Anti-inflammatory activity – Inhibition of Cyclooxygenase-1 enzyme (COX-1)
Background: Today, consumers seek natural alternatives to synthetic pharmaceutical products to aid with a variety of ailments experienced during daily life. Thus, dietary food supplements containing natural substances such as gingko biloba, ginseng, and others have recently been marketed for a variety of purposes. Pain and inflammation are commonly treated by the use of aspirin, ibuprofen (Motrin®, Advil®), and other similar substances commonly known as NSAIDs. Inflammation is caused, in part, by a class of compounds known as prostaglandins, which are released by a host in response to mechanical, thermal, chemical, bacterial, and other insults (Moncada et al., Handbook of Exp. Pharm. Vol 50-1, Springer Verlag, pp 588-616, 1978; Samuelsson, Science, 220: 568-575, 1983; Davies et al, Ann. Rev. Immunol. 2:335-357, 1984). Prostaglandin synthesis is accomplished in a stepwise manner by a ubiquitous complex of microsomal enzymes. The first enzyme in this biosynthetic pathway is prostaglandin endoperoxide synthase. This enzyme also is also known as fatty acid cyclooxygenase (COX). There are two isoforms of this enzyme known as cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), respectively (Smith, Am. J. Physiol., 268:F181-F191, 1992). COX inhibition has varied pathophysiological roles in different tissues and animals that can translate into different outcomes owing to inhibition from ns- and s-NSAIDs. COX inhibition in the gastrointestingal tract (GI) and in the musculoskeletal, ocular, and renal systems, the following outcomes were seen: (1) COX-2 s-NSAIDs have an improved GI tolerability profile when compared to ns-NSAIDs; (2) preclinical models reveal that effects of COX-2 s-NSAIDs and ns-NSAIDs on bone, tendon, and ligament healing are variable; (3) COX-2 s-NSAIDs and ns-NSAIDs have generally beneficialeffects in the ocular system; and (4) some COX inhibition renal effects, such as handling of electrolytes, can be predicted from preclinical animal models.
The COX-1 inhibition assay was performed on the 06/08/2020.
Figure 2: Inhibition of the COX-1 enzyme by meat snax products.

Result: The meat snax products were assayed at concentrations of 1 mg/ml in triplicate (n=3) and results are shown in Figure 2. Inhibition of COX-1 ranged from 49.29 % for the seaweed containing treat to 58.38 % for the joint aid product when samples were assayed at a concentration of 1 mg/ml, respectively.
Assay 3: Dipeptidyl peptidase IV inhibition (DPP-IV inhibition)
Background: Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) fulfill the definition of an incretin hormone in humans. Incretins have potential therapeutic use as anti-hyperglycemic agents, receiving much attention as a potential new form of treatment for diabetes, especially against Type 2 diabetes mellitus (T2DM) originally, and more recently, T1DM. However, the therapeutic potential of endogenous GLP-1 and GIP (incretins) is limited, because of rapid inactivation by dipeptidyl peptidase-4 (DPP-4). DPP-4 inhibitors and DPP-4 resistant GLP-1 analogs, have emerged as new classes of antihyperglycemic agents. Sitagliptin is such a drug which is commercially available and is used to treat diabetes in humans.
Sitagliptin is a dipeptidyl peptidase-4 inhibitor aimed at treating Type 2 diabetes mellitus (T2DM) and T1DM, by increasing blood levels of Glucagon-like peptide 1 (GLP-1) and insulin. By preventing GLP-1 and GIP inactivation, both incretins are able to increase insulin secretion, decrease gastric acid secretion and decrease blood glucose levels. Previously a study to characterize Sitagliptin’s ability for glycemic control, in healthy dogs under an oral glucose tolerance test (OGTT) environment was carried out. Overall, Sitagliptin did not result in any significant changes to temporal glucose and insulin concentrations. However, a ~55% increase in median total GLP-1 AUC0–120min was observed, as compared to baseline control in healthy dogs (n=5), thus indicating a similar mode of action of Sitagliptin between healthy dogs and humans (Oda et al., 2014). The aim of this assay was to identify if meat snax products could inhibit DPP-IV when assayed at a concentration of 1mg/ml compared to the positive control and commercial drug Sitagliptin.
This assay was carried-out on the 5/10/2020.
Figure 3: Dipeptidyl peptidase IV inhibition by meat snax products (n=2)

Results: As shown in Figure 3, the meat snax products when assayed at a concentration of 1 mg/ml inhibited DPP-IV by between 72.74 % (seaweed inclusive snack) and 87.61% (Antiworm snack) compared to sitagliptin, which, inhibited DPP-IV by 98.1 % when assayed at a concentration of 100 mM. This is a very positive result and warrants further investigation perhaps in in vivo trials. The next step is to determine the concentration of the snack that gives 50% inhibition (IC50 value).
2. Nutritional analysis
2.1 Protein & Lipid
The total protein content of the samples was determined using the Dumas combustion method using a LECO FP328 Protein analyser (LECO Corp, MI USA), according to Association of Official Analytical Chemists (AOAC) method 992.15 (AOAC, 1990). The conversion factor of 6.25 was used to convert total nitrogen to protein. The total fat content was determined gravimetrically using Ankom XT15 Extractor (Ankom Technology, Macedon NY, USA) for lipid extraction, after previous acid hydrolysis using Ankom HCI Hydrolysis System according to manufacturers’ operating manual.
- Ash
The ash content was determined gravimetrically, as previously described (Kolar, 1992). Briefly, ash content of all the samples was determined using the Muffle furnace. Samples were weighed in dried and marked crucibles. Before the ashing procedure, samples were initially charred by initiating a pre-burn process on a hotplate for 3 h at 100 ºC. The pre-burned samples were carefully transferred to a muffle furnace for ashing overnight at 540 ºC. After ashing was complete, samples were cooled to room temperature in a desiccator, and the weight of the % ash was calculated using the following formula:

Where: M0 = mass in grams of dish; M1 = mass in grams of dish and test portion; M2 = mass in grams of dish and ash.
2.3 Amino acid analysis
Determination of the total amino acid composition of samples was done by further hydrolysing samples using 6 M HCL at 110˚C for 23 h (Fountoulakis & Lahm, 1998). The samples were then de-proteinized by mixing equal volumes of 24% (w/v) tri-chloroacetic acid and sample. These were allowed to stand for 10 min at room temperature before centrifugation at 14,400 x g for 10 min. The supernatants were removed and diluted with 0.2 M sodium citrate buffer, pH 2.2 to give approximately 250 nmol of each amino acid residue. Samples were then diluted 1:2 with the internal standard nor-leucine to give a final concentration of 125 nm/ml. Amino acids were quantified using a Jeol JLC-500/V amino acid analyser (Jeol Ltd., Garden city, Herts, UK) fitted with a Jeol Na+ high performance cation exchange column.
- Results – Nutritional analysis

2.4.1 Lipid content of pet treats (Meat Snax)
The lipid or fat content of the meat snax pet treats ranged from 6.06 (Sample 2)- 7.70 (Sample 3)%. The lowest lipid content was observed for meat snax product sample 2 (Dental treat).
2.4.2 Protein content of Meat Snax pet treats
The protein content of all Meat Snax products were in the range of 47-49 % protein based on dry weight of the product. The Association of American Feed Control Officials requires adult dog food to contain a minimum of 18 % protein on a dry matter basis. These treats are well within the recommendations.
2.4.3 Ash content of Meat Snax pet treats
The ash content of MeatSnax products ranged from 7.41 (Sample 4) to 8.56 % (Sample 1). Salts are usually a major constituent of seaweed ingredients, with the main sources being the seawater. Levels of ash are within those permitted in dog pet treats. The average ash content in commercial pet food/treats is 5-8 % of dry weight.
